

|

|
Theme 1 : Biosensors |
Pierre Vincent; Elvire Guiot | |
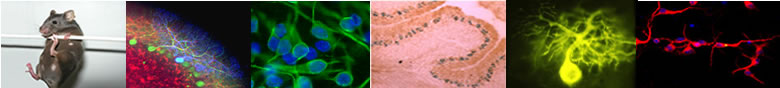
Genetically-encoded biosensors allow real-time imaging of specific intracellular events in living cells. Epac-based sensors (Ponsioen; DiPilato; Nikolaev) report changes in cAMP concentration and AKAR-type biosensors (Zhang)
are reporting the PKA/phosphatase equilibrium. These biosensors are
expressed in brain slice preparations using viral vectors and imaged in
real-time with wide-field (see movie) or two-photon microscopy. |
Collaborations: |
Theme 2 : Spatial and temporal integration of the cAMP/PKA signal |
Elvire Guiot; Liliana Castro; Danièle Tritsch | |
Two
photon microscopy provides superior spatial resolution allowing us to
monitor signal propagation throughout the dendritic tree (figure), while
electrophysiology tells us about PKA's effect on membrane channels. |
Theme 3 : Control of the cAMP/PKA signal |
Collaborations: |
Theme 4 : Functional role in vivo |
Pierre Vincent | |
The
cellular approach on brain slice preparations will be paralleled by
recordings performed in vivo. In collaboration with Mauna-Kea
technologies, fibered fluorescence imaging allowed us to record for the
first time calcium signals from neurons located in deep brain regions (Vincent, 2006). |
Collaborations: |